Chimeric antigen receptor (CAR) T cell therapies have transformed the treatment of hematologic malignancies, leading to FDA approval in B-cell acute lymphoblastic leukemia, non-Hodgkin lymphoma, and multiple myeloma. While many patients with previously untreatable disease have achieved excellent responses and long-term remissions, a substantial number do not respond or relapse after months to years. Most prior studies of CAR T cell-intrinsic determinants of response and resistance have focused on infusion products. Functional expansion and persistence of CAR T cells in patients constitute an important feature of treatment success, yet the trajectories of CAR T cells and their molecular underpinnings remain poorly understood due to technical challenges.
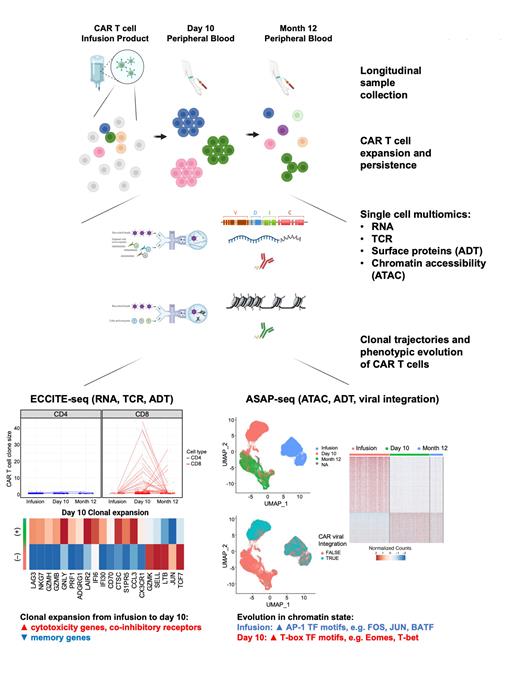
Here we performed single-cell multiomics with T-cell receptor (TCR) lineage tracing to study the molecular attributes of CAR T cell fitness, which we define as the ability to expand and/or persist to at least 12 months after infusion. We recently published long-term follow-up from a prospective single-center phase 2 clinical trial in which CD19-directed CAR T cells with 4-1BB co-stimulation were administered to patients with relapsed or refractory chronic lymphocytic leukemia (CLL) not in complete remission despite at least 6 months of ibrutinib. In this trial, we found high rates of deep and durable remissions with prolonged persistence of functional CAR T cells determined by flow cytometric detection of CAR T cells with concomitant B-cell aplasia. We obtained CAR T cell infusion products and peripheral blood at day 10 and month 12 from five patients with durable remissions. We then performed single-cell multiomics sequencing to measure gene expression, TCRs, surface proteins, and chromatin accessibility to define the multidimensional phenotypes and clonal trajectories of CAR T cells over time ( Figure).
We first evaluated CAR T cell dynamics from infusion product to day 10 and identified clones that expanded using TCR as a molecular barcode. While ~90% of CAR T cells in the infusion product are CD4 +, peak expansion at day 10 is driven by effector CD8 + CAR T cells. Clonally expanded CAR T cells at day 10, compared to non-expanded CAR T cells, showed higher expression of multiple cytotoxicity genes ( GZMH, GZMB, GNLY, PRF1) and co-inhibitory receptors ( LAG3, PDCD1, and KLRB1) and lower expression of memory genes ( SELL, LTB, TCF7). Using TCR lineage tracing and a machine learning classifier, we identified similar markers predictive of clonal expansion from the infusion product, e.g. CD8A, GNLY, KLRG1, KLRK1 among the top positive markers and CD4 and SELL among the top negative markers.
Along with clonal expansion, we found that CD8 + CAR T cells undergo significant evolution in their chromatin accessibility landscape from infusion product to day 10. Genomic regions that are more open at infusion are enriched for Fos/Jun (AP-1) and Batf family of transcription factor (TF) motifs, consistent with their function in promoting effector T cell differentiation and proliferation. Genomic regions that are more open at day 10 are enriched for Eomes, T-bet, and other T-box TF motifs, consistent with their roles in regulating T cell cytolytic function, memory formation, and exhaustion. These data provide insights into the regulatory mechanisms of rapid CAR T cell expansion post-infusion in patients which have not yet been reported.
We found that most clonally expanded CAR T cells at day 10 contracted or disappeared by month 12. However, a subset of CD8 + CAR T cells in 2 patients that did not undergo early expansion surprisingly demonstrated late clonal expansion and persistence at month 12, coinciding with hyper-expansion (>200 cells per clone) of their TCR clonotypes with or without CAR expression. We then investigated the specificity of TCRs using publicly available databases and found late expansions of clones predicted to bind viral peptides. This observation suggests that the TCR antigen specificity may contribute to the long-term persistence or fitness of CAR T cell clones, in contrast to recent studies of long-lived CAR T cells in a limited number of patients which argue that persistence is driven by specific phenotypes rather than clonotype-specific features. Further validation of this finding is ongoing through flow enrichment of rare persistent CAR T cells for deeper single-cell sequencing and in vitro cellular immunology assays.
Disclosures
Porter:Janssen: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Mirror Biologics: Membership on an entity's Board of Directors or advisory committees; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Sana Therapeutics: Consultancy, Current equity holder in publicly-traded company; Tmunity: Patents & Royalties; Wiley and Sons Publishing: Honoraria; Genentech: Current equity holder in publicly-traded company; DeCart: Membership on an entity's Board of Directors or advisory committees; Capstan Bio: Honoraria; BMS: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Angiocrine Bio: Membership on an entity's Board of Directors or advisory committees. Mustjoki:BMS: Honoraria, Research Funding; Pfizer: Research Funding; Novartis: Honoraria, Research Funding; Dren Bio: Honoraria. Gill:Kite Pharma: Consultancy; Carisma Therapeutics: Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: patents, Research Funding; Interius Biotherapeutics: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Asher: Research Funding; Currus: Membership on an entity's Board of Directors or advisory committees; Inndura: Membership on an entity's Board of Directors or advisory committees; Mission Bio: Membership on an entity's Board of Directors or advisory committees; NKILT: Membership on an entity's Board of Directors or advisory committees; Vor Bio: Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal